A Breakthrough in Intestinal Healing.
A pioneering approach to intestinal lengthening that harnesses the body’s natural ability to grow new bowel in patients with Short Bowel Syndrome (SBS).
A Feasibility Study to Evaluate Safety and Probable Benefit of the Eclipse XL1 System for Distraction Enterogenesis in Adult and Pediatric Patients with Short Bowel Syndrome.
The Eclipse XL1 System is being studied for intestinal lengthening in pediatric and adult patients with Short Bowel Syndrome.
Eclipse XL1 System
The Eclipse XL1 Implant
Specialized coil for intestinal lengthening.
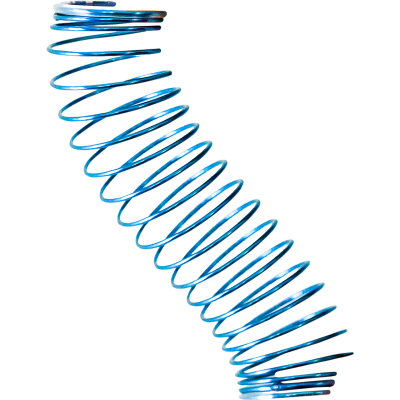
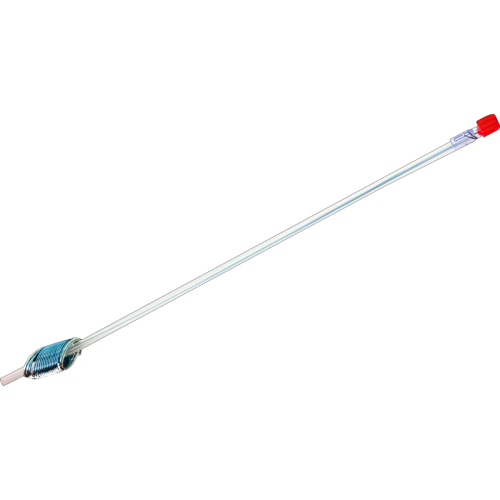
Eclipse XL1 System
The Eclipse XL1 Delivery Device
XL1 implant is loaded onto the delivery system for easy deployment in a surgical setting.
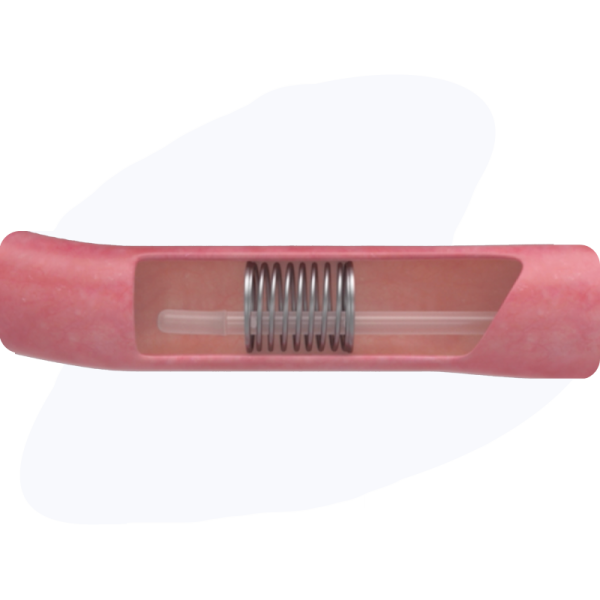
Enterogenesis (Mechanical Lengthening) Procedure
Step 01
Compressed XL1 is introduced inside the intestine surgically.
Enterogenesis (Mechanical Lengthening) Procedure
Step 02
The XL1 coil ends are sutured in place.
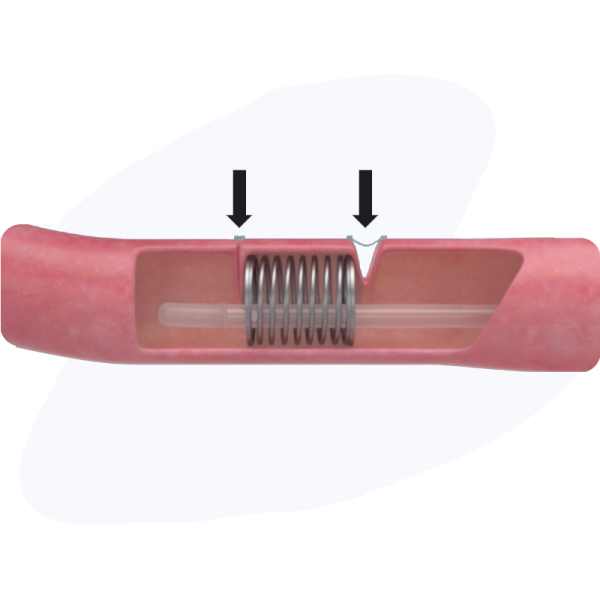
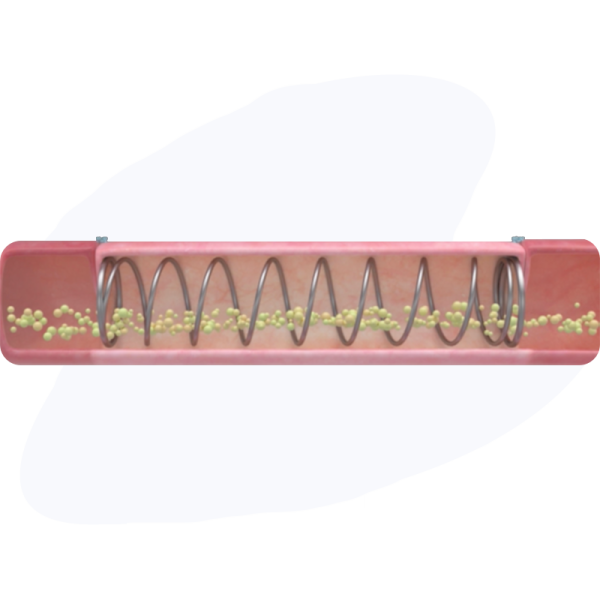
Enterogenesis (Mechanical Lengthening) Procedure
Step 03
Coil applies gentle pressure to the intestine over a 2-3 week period, stimulating new intestinal growth and lengthening the treated region of the intestine.
Enterogenesis (Mechanical Lengthening) Procedure
Step 04
Implant will be removed at subsequent surgical procedure or, in some cases, can pass naturally through the GI tract once sutured ends dissolve – roughly a 1-3 month time period.
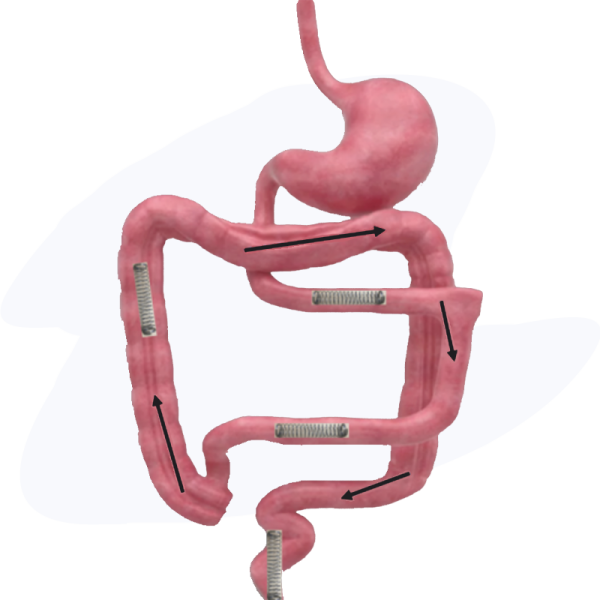
Intended Treatment Location.
There is potential for intestinal lengthening throughout the GI tract with the current focus and indication for small intestine treatment in patients with short bowel syndrome. Study treatment may occur throughout the small intestine with a large area of focus being on increasing the length of the ileum given its importance in digestive function.
Eligibility Criteria for Study Participation.
To ensure safety and consistency in evaluating the Eclipse XL1 System, this study includes carefully defined eligibility requirements. Participants must meet key inclusion criteria and not fall under any of the exclusion conditions listed below. These guidelines help determine whether a patient is an appropriate candidate for this investigational treatment.
Patient Inclusion Criteria
Subject has short bowel syndrome, defined as 50% or less of expected bowel length based on subject age and/or height, and measured at the time of the subject’s prior intestinal resection.
Male or female patients aged 12 months to 65 years inclusive.
The parent or legal guardian of the subject is able to read, understand, and is willing to provide informed consent (and the subject is able to assent, if over 12 years of age).
The subject or parent or legal guardian of the patient is able to understand the requirements of the study and is willing to bring the subject to all clinic visits and complete all study related procedures (as determined by the investigator).
Patient Exclusion Criteria
Learn More About the Study
Full details about this clinical trial, including participating sites, eligibility criteria, and enrollment status, are available on the official ClinicalTrials.gov listing.
For More Information
Get In Touch
Have any questions or looking for more information? Contact us at info@eclipseregenesis.com.
Healing from Within Starts Here
We believe the future of gastrointestinal care is regenerative, not restrictive. With the Eclipse XL1, we’re working to give patients the chance to grow what was once gone, and to live fuller, more independent lives.